
Zhao Yu, Liu Xinlei, Li Yihao, Xu Leichuan, Su Yanhao, Jiang Jiazhen, Wang Ming'an. Simple and Cost-Effective Synthesis of Fmoc-DOPA(acetonide)-OH Liu Jingjing, Zhang Donghui, Jiang Weinan, Liu Runhui. New Method for the Synthesis of 2,5-Diaryl Substituted Thiazoles Synthesis and Cytotoxicity Evaluation of Dehydroabietic Acid Derivatives Bearing Nitrate Moiety Li Fangyao, Huang Lin, Zhou Xiaoqun, Li Qian, Ma Xianli, Duan Wengui, Wang Xiu. Pan Chao, Liu Peng, Wu An'guo, Li Ming, Wen Lirong, Guo Weisi.Įlectrochemical-Promoted Synthesis of 2-Thiazolines via Selenylation/Cyclization of N-Allylthioamides Liang Wei, Tan Chengxia, Weng Jianquan, Liu Xinghai.Īdvances on Synthesis and Biological Activities of Mesoionic Compounds Synthesis and Insecticidal Activity of Novel meta-Diamide Compounds Containing Cyclopropyl Group Luo Chunyan, Ma Wenjing, Lv Liang, Pang Huailin, Xiang Juncheng, Zhou Liqi, Yin Dulin, Liu Jiyong. Lei Kang, Li Pan, Zhou Xiaoyun, Wang Shiben, Wang Xuekun, Ji Lusha, Liu Renmin, Xu Xiaohua.ĭesign, Synthesis and Herbicidal Activity of 5-Acylbarbituric Acid Derivatives and Study of Molecular Mode of Action Synthesis and Antitumor Activity of Novel Quinazoline Derivatives Containing Acrylamide Zhang Luye, Zhang Yang, Wang Zhengjie, Wang Tao, Li Erdong, Liu Limin, Liu Xiujuan, Zheng Jiaxin, Ke Yu, Shan Lihong, Liu Hongmin, Zhang Qiurong. Zhang Jingpeng, Yang Zhaokai, Qin Yaoguo, Yang Xinling.ĭesign, Synthesis and Biological Activity of ( E)- β-Farnesene Analogues Containing 1,2,3-Thiadiazole
Nitration (Nitroalkylation) of Pheophorbide and Synthesis of Chlorophyllous Chlorin Derivatives Zhang Zhu, Li Jiazhu, Wang Xinyue, Ma Jihua, Wang Xu, Wang Jinjun.
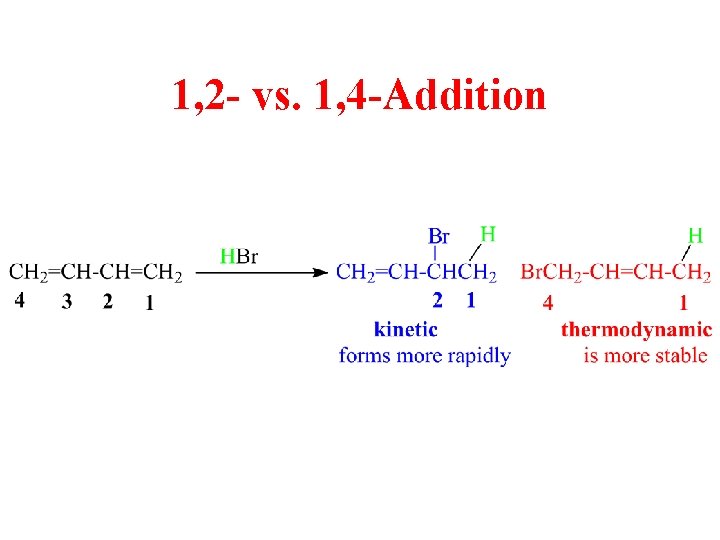
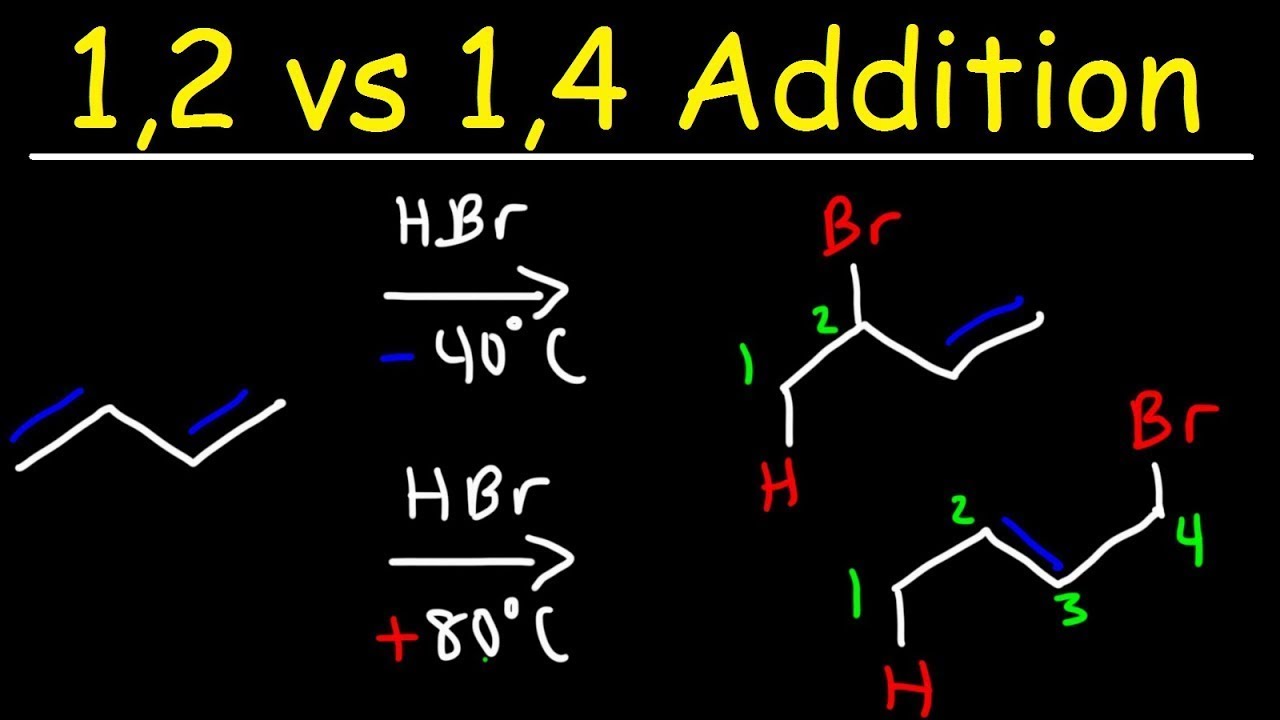
Selectfluor as “Fluorine-Free” Functional Reagent Applied to Organic Synthesis under Transition Metal-Free Conditions Kong Yaolei, Sun Xiaotong, Weng Jianquan. Recent Progress on Quinone Imine Ketals: Synthesis and Applications Key words: 2-cycloenone, synthesis, dialkylphenylsilyl cuprate, 1,4-addition, 2-substituted-3-silylcycloalkanone, stereoselectivity All products were characterized by IR, 1H NMR, 13C NMR, MS and HRMS spectra. It was also observed that arylmethyl bromides reacted with enolates resulting from 1,4-addition of dimethylphenylsilyl cuprate to 2-cycloenones giving exclusively trans isomers. However, it was first found that the addition gave very high trans selectivity independent of the aryl group, ring size and substituents on the silicon atom by quenching the reactions with methanol at room temperature. It was found that the stereoselectivity of the 1,4-addition of dialkylphenylsilyl cuprates to 2-aryl-2-cycloenones depended on the nature of aryl group and ring size by quenching the reactions with saturated NH 4Cl solution at 0 ℃.

1 2 1 4 ADDITION SERIES
The stereoselectivity of 1,4-addition of dialkylphenylsilyl cuprates to 2-cycloenones was investigated and meantime a series of new 2-substituted-3-silylcycloalkanones were synthesized.


 0 kommentar(er)
0 kommentar(er)
